Fort Detrick, MD –
When the Defense Department (DOD) needs to develop tools and equipment to protect the Joint Force, it is critical that these items are put through rigorous testing and validation measures to ensure that they have the proper capabilities to beat the evolving threat. The Defense Biological Product Assurance Office (DBPAO), which is a part of the Joint Program Executive Office for Chemical Biological Radiological and Nuclear Defense’s (JPEO-CBRND) Joint Project Lead for CBRND Enabling Biotechnologies (JPL CBRND EB), operates as the principal resource of high quality, validated, and standardized biological reference materials, reagents, and assays, which ensures that the DOD is able to perform the best testing by providing quality tools.
These testing supplies meet the technology development and sustainment needs of the DOD and its partners. The DBPAO also supports the biological defense community’s mission by facilitating the transition of new technologies and coordinating their advanced development, efficient production and distribution.
JPL CBRND EB is working on developing a new, third detection method using bacteriophage to offer additional detection capability to Warfighters.
“We are constantly looking for ways to improve our Warfighters’ capabilities by increasing the ability to detect threats quickly,” said Bryan Necciai, Director of DBPAO.
Currently, JPL CBRND EB offers two lines of detection products and reagents for biological agents: molecular assays and immunoassays. Molecular assays, such as real time polymerase chain reaction (PCR), which became a household name during the COVID-19 pandemic, detect genomic targets such as RNA and DNA, while immunoassays detect antigens, which are usually proteins on the surface of the pathogen itself. Molecular assays are very sensitive and can detect the presence of even a few copies of the targeted genetic material.
Immunoassays are less sensitive than molecular assays and are often preferred for protein-based detection of biothreats, like toxins. Immunoassays are available in an easy-to-use format such as in lateral flow immunoassays (LFIs), based on the technology in at-home pregnancy tests. LFIs are also relatively inexpensive, making them an ideal tool to screen quickly for the presence or absence of a pathogen.
However, these assays also have their limitations. While both assay types can identify the presence of a biothreat agent in a sample, they cannot identify whether that agent is alive. Both assay types are often employed to increase detection confidence for biothreats, with LFIs used as a screening tool in the field, followed by laboratory confirmation with PCR assays. Many of us are all too familiar with this practice from our own experiences using COVID-19 at-home LFIs kits, followed up with PCR confirmatory tests after we receive LFI results to obtain a more conclusive result and aid in decision making to prevent further spread.
To further expand biothreat detection capability, JPL CBRND EB is working on a third detection paradigm using bacteriophage, sometimes referred to as ‘phage’-based biothreat detection in addition to PCR assays and LFIs.
Every bacterial species is infected with phages, making phages one of the most abundant organisms on earth. Phages are species-specific and often strain-specific, making them unique targets or reagents for detection platforms. There are numerous advantages of using phages in detection and/or diagnostics, including their ability to distinguish between related bacterial strains, ability to be engineered to detect agents other than bacteria, potential to distinguish the presence of live pathogen in a sample, and the availability of an inexhaustible pool of naturally occurring phages to easily identify newly emergent and unknown pathogens.
While phage-based detection efforts offer these capabilities, their true potential has not yet been realized in biothreat detection. Currently, phage-based detection is far less common than molecular or immunoassay-based detection platforms. JPL CBRND EB is working with our partners to develop future phage-based detection tools with the goal of offering these enhanced capabilities to the joint force.
These phage-based detection reagents can be provided with easy-to-use fluorescence detectors such as UV pens for use in far-forward applications. These novel phage reagents add depth and breadth to JPL CBRND EB’s product portfolio and provide additional forward testing strategies to increase confidence in in bio detection results. They can also be used as tools for laboratory research.
“DBPAO is proud to support the Joint Force by enhancing the way threats are detected,” said Bryan Necciai, Director of DBPAO. “We look forward to adding phage-based detection tools to our robust portfolio of biothreat detection products.”
This effort from JPL CBRND EB is just one example of the many ways teams across the JPEO-CBRND foster innovative solutions to protect our joint force and the nation from biothreats. Modernizing the DOD’s approach across the CBRN medical countermeasure spectrum by building, maturing, and integrating capabilities and infrastructure to support accelerated development, manufacturing, testing, and deployment allows us to protect, diagnose, and treat our warfighters efficiently and effectively, keeping them safer in CBRN contested environments.

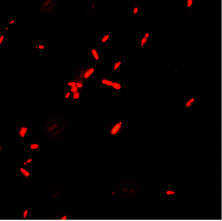
 Left: Bacillus anthracis Sterne bacteria with AP50c phage particles attached on the surface (Magnification 25000X).
Left: Bacillus anthracis Sterne bacteria with AP50c phage particles attached on the surface (Magnification 25000X).
Center: Bacillus anthracis bacteria bound by red fluorescent phage protein.
Right: Bacillus anthracis Sterne bacteria with AP50c phage particles attached on the surface (Magnification 15000X).