The Joint Project Manager for Chemical, Biological, Radiological, and Nuclear Medical (JPM CBRN Medical) aims to provide U.S. military forces and the Nation with safe, effective, and innovative medical solutions to counter chemical, biological, radiological, and nuclear (CBRN) threats. The JPM CBRN Medical facilitates the advanced development and acquisition of medical countermeasures (MCM) and systems to enhance the Nation’s biodefense response capability.
Overview
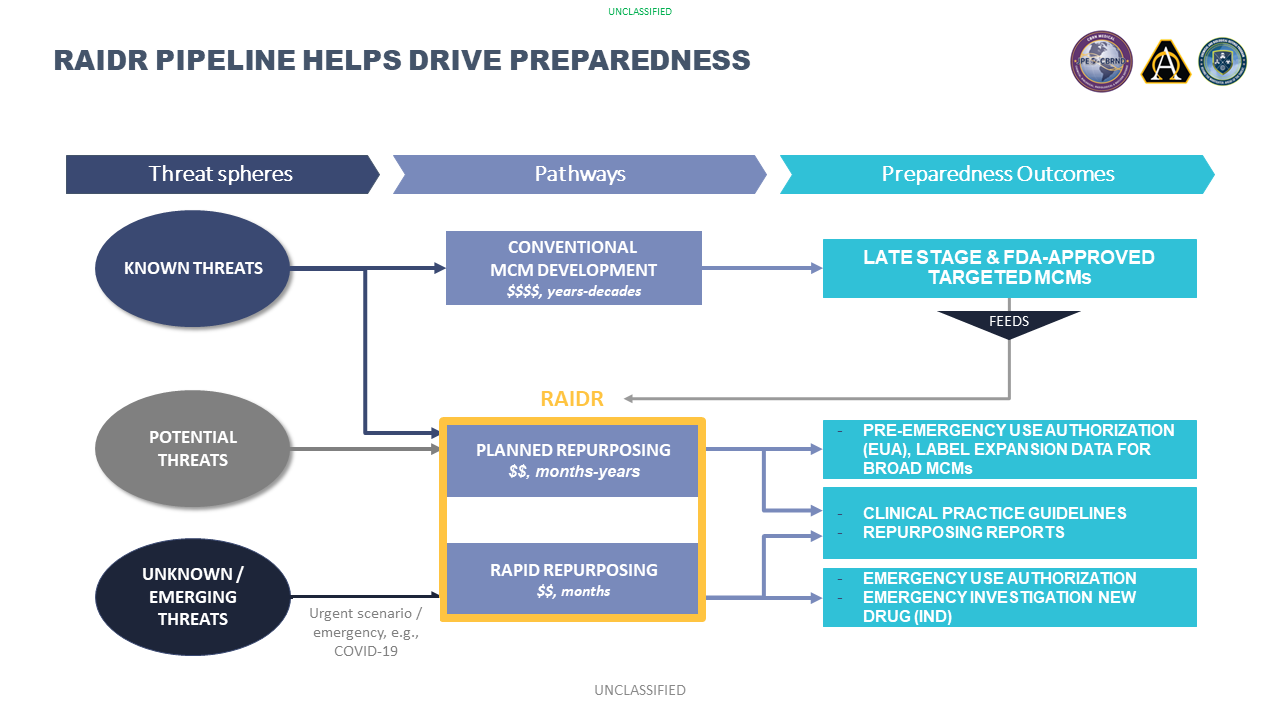
The mission of the CET RAIDR program is to provide the U.S. military forces and the Nation with safe, effective, and affordable prophylactic and therapeutic solutions to counter traditional, emerging, and engineered/synthesized biological and chemical threats. The CET RAIDR program is based on an Office of the Secretary of Defense (OSD)-directed requirement, indicating that repurposing will be an integral pillar for future MCM development. From a strategic perspective of national preparedness, RAIDR supports the realization the 2021 American Pandemic Preparedness Plan: Transforming Our Capabilities. Additionally, the 2022 National Biodefense Strategy and Implementation Plan calls for repurposed therapeutics within reach for pandemic preparedness and national security. This cannot be achieved without considering appropriate, relevant, repurposing of available pharmaceuticals. This 2022 plan directs stakeholders to “identify, develop, test, authorize, manufacture, and deploy new and repurposed therapeutics”. This goal synergistically maps to the CET RAIDR programs mission space.
 The impact of CET RAIDR is illustrated by the partnership with Rigel Pharmaceuticals to repurpose TAVALISSE (fostamatinib), posturing this product for the potential use in the treatment of acute respiratory distress syndrome (ARDS) associated with COVID-19. This partnership demonstrates the utility of repurposing FDA-approved countermeasures to rapidly address medical capability gaps in support of the warfighter.
The impact of CET RAIDR is illustrated by the partnership with Rigel Pharmaceuticals to repurpose TAVALISSE (fostamatinib), posturing this product for the potential use in the treatment of acute respiratory distress syndrome (ARDS) associated with COVID-19. This partnership demonstrates the utility of repurposing FDA-approved countermeasures to rapidly address medical capability gaps in support of the warfighter.